Airplane on a Conveyor Belt
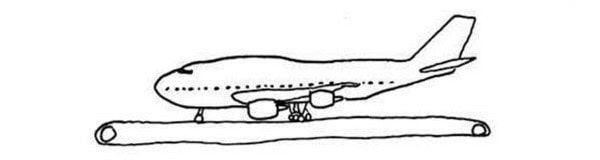
A Boeing 747 is sitting on a conveyor belt, as wide and long as a runway. The conveyor belt is designed to exactly match the speed of the wheels, moving in the opposite direction. Will the plane take off?
We do not know where this puzzle originated from. If you have any information, please let us know via email.
A Boeing 747 is sitting on a conveyor belt, as wide and long as a runway. The conveyor belt is designed to exactly match the speed of the wheels, moving in the opposite direction. Will the plane take off?

A man was going to bleach his socks because they had gotten muddy the day before. As he was pouring the bleach into the washing machine, he spilled some on the floor. He got some cleaning fluid and mopped it up with a rag. Minutes later he was dead. What killed him?
If you pull straight back on a pedal of a bicycle when it is at its lowest position, will the bicycle move forward or backward?

You have a glass of water and an ice cube floating in it. When the ice cube melts, will the water level increase, decrease or remain the same?
NASA was considering sending canaries into space to study them under zero gravity. The project was scrapped when someone realized that in spite of having sufficient water supplies, they could die of dehydration within a few hours. Why?
You wake up on a frozen lake in an isolated region, a hundred meters away from the shore. The surface of the lake is frictionless, and no grip of any kind can be attained over it. You find just your mobile phone in your pocket, but when you take it out to call for help, you realize there is no reception.
If there is no wind force to help you escape, what are you going to do to avoid freezing to death?
How much will a 12° angle measure when looked at under a microscope that magnifies 8 times?
You are sitting in a motionless car, which is tightly sealed, i.e. no open windows, holes in the car, etc. A helium balloon on a string is tied to the floor. If you start accelerating the car, is the balloon going to move back, forward, or stay in place?
When a car is making a turn, are its front wheels parallel to each other?
You are shown two cylinders – one of them is made of gold and hollow, the other one is made of alloy and solid. Both of them have the same weight, shape, and are colored in black. How can you figure out which cylinder is the golden one, without scratching the paint?
Please confirm you want to block this member.
You will no longer be able to:
Please allow a few minutes for this process to complete.
Notifications